Medical
Ensure the reliability of your medical products
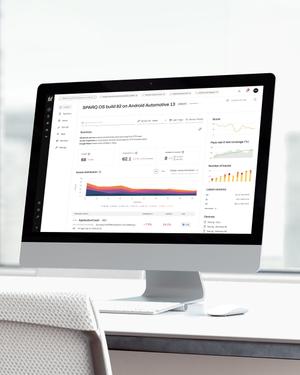
Our modern-era software analytics methodologies help you discover and fix software defects affecting long-term product reliability, while systematically collecting FDA objective evidence.
Advantages
FDA objective evidence
Collect the objective evidence data for regulatory agencies automatically during QA runs; your documentation proof will be complete and nothing is missing.
E2E scope
By including external devices and backend systems in the analysis scope, you avoid nasty integration surprises that often occur close to product launch, or when the device is already on markets.
Continuous monitoring
You get constant quality feedback on your latest SW builds: how well do you meet the requirements, and is there any regression happening?
CI/CD integration
The analytics suite, integrated with your CI/CD system, provides immediate developer feedback when a change is made. This enhances the overall quality of your software baselines, and speeds up development cycles.
Global audiences
Your customer base is global, and so is our approach: all language variants are included in QA scope.
Cost savings
Automated regulatory data and quality monitoring saves money, as the amount of manual work is reduced and the probability of human errors goes down.
Sign up for our newsletter
Subscribe for periodic emails about product updates, events, and developments